Find verified Venetoclax API manufacturers, suppliers, and distributors across the globe. Explore companies that offer high-purity Venetoclax for pharmaceutical use, along with product details, certifications, and reliable sourcing options. Compare prices, check availability, and connect directly with trusted suppliers for Venetoclax API.
Alternate Names: Venclexta, venclyxto, venetoclaxum
CAS No: 1257044-40-8
PubChem CID: 49846579
Mol Formula: C45H50ClN7O7S
Mol Weight: 868.4 g/mol
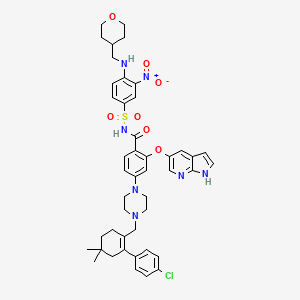
IUPAC Name: 4-[4-[[2-(4-chlorophenyl)-4,4-dimethylcyclohexen-1-yl]methyl]piperazin-1-yl]-N-[3-nitro-4-(oxan-4-ylmethylamino)phenyl]sulfonyl-2-(1H-pyrrolo[2,3-b]py -Show More
API Description: Venetoclax is a BCL-2 inhibitor that was initially approved by the FDA in April 2016. Proteins in the B cell CLL/lymphoma 2 (BCL-2) family are important regulators of the apoptotic (programmed cell death) process,. Venetoclax is used to treat chronic lymphocytic leukemia (CLL) and certain types of small lymphocytic lymphoma. CLL is the most prevalent leukemia diagnosed in Western countries. Venetoclax was developed through reverse engineering of the BCL-2 protein family inhibitor, navitoclax. Venetoclax is approximately 10 times more potent than navitoclax with regard to induction of apoptosis in CLL cells. A new indication was approved in 2018 for the treatment patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), with or without 17p deletion, who have received at least one prior therapy. Previously, this drug was indicated only for patients with 17p gene deletion.