Find verified Paliperidone API manufacturers, suppliers, and distributors across the globe. Explore companies that offer high-purity Paliperidone for pharmaceutical use, along with product details, certifications, and reliable sourcing options. Compare prices, check availability, and connect directly with trusted suppliers for Paliperidone API.
Alternate Names: 9 Hydroxy risperidone, 9 Hydroxyrisperidone, 9 OH risperidone, 9-hydroxy-risperidone, 9-hydroxyrisperidone, 9-OH-risperidone, Invega, Invega Sustenna, paliperidone, paliperidone palmitate
CAS No: 144598-75-4
PubChem CID: 115237
Mol Formula: C23H27FN4O3
Mol Weight: 426.5 g/mol
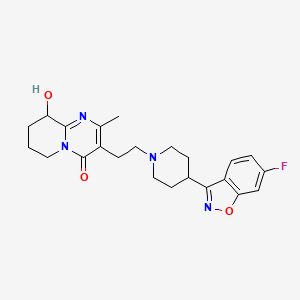
IUPAC Name: 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-9-hydroxy-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one
API Description: Paliperidone is an atypical antipsychotic medication used to treat schizophrenia and schizoaffective disorder. It works mainly by blocking dopamine (D₂) and serotonin (5-HT₂A) receptors and is the active metabolite of risperidone.