Find verified Linaclotide API manufacturers, suppliers, and distributors across the globe. Explore companies that offer high-purity Linaclotide for pharmaceutical use, along with product details, certifications, and reliable sourcing options. Compare prices, check availability, and connect directly with trusted suppliers for Linaclotide API.
Alternate Names: Linzess, Constella, Linaclotida
CAS No: 851199-59-2
PubChem CID: 16158208
Mol Formula: C59H79N15O21S6
Mol Weight: 1526.8 g/mol
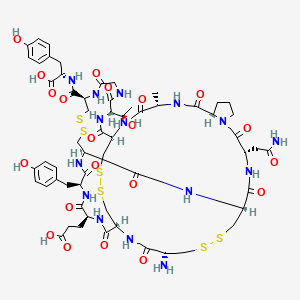
IUPAC Name: (2S)-2-[[(1R,4S,7S,13S,16R,21R,24R,27S,30S,33R,38R,44S)-21-amino-13-(2-amino-2-oxoethyl)-27-(2-carboxyethyl)-44-[(1R)-1-hydroxyethyl]-30-[(4-hydroxyph -Show More
API Description: Linaclotide is a synthetic 14-amino acid cyclic peptide and first-in-class guanylate cyclase-C (G-CC) agonist. Linaclotide is structurally related to human guanylin and uroguanylin, paracrine peptide hormones that are endogenous activators of GC-C. It is also a homolog of a heat-stable enterotoxin derived from Escherichia coli, the first natural ligand that activates GC-C. Linaclotide is used for the treatment of various types of constipation, including irritable bowel syndrome with constipation.