Find verified Gilteritinib API manufacturers, suppliers, and distributors across the globe. Explore companies that offer high-purity Gilteritinib for pharmaceutical use, along with product details, certifications, and reliable sourcing options. Compare prices, check availability, and connect directly with trusted suppliers for Gilteritinib API.
Alternate Names: ASP-2215, gilteritinibum
CAS No: 1254053-43-4
PubChem CID: 49803313
Mol Formula: C29H44N8O3
Mol Weight: 552.7 g/mol
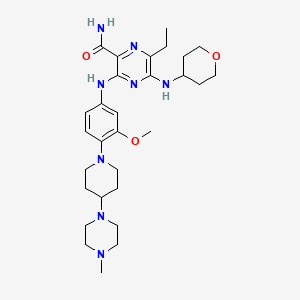
IUPAC Name: 6-ethyl-3-[3-methoxy-4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]anilino]-5-(oxan-4-ylamino)pyrazine-2-carboxamide
API Description: Gilteritinib, also known as ASP2215, is a small molecule part of the FLT3 tyrosine kinase inhibitors that presented a greater selectivity and potency when compared with other agents from this group. It is a pyrazinecarboxamide derivative that showed high selectivity to FLT3 preventing the c-Kit -driven myelosuppression observed in other therapies. Gilteritinib was developed by Astellas Pharma and FDA approved on November 28, 2018. This drug was approved after being designed as an orphan drug with a fast track and priority review status