Find verified Febuxostat API manufacturers, suppliers, and distributors across the globe. Explore companies that offer high-purity Febuxostat for pharmaceutical use, along with product details, certifications, and reliable sourcing options. Compare prices, check availability, and connect directly with trusted suppliers for Febuxostat API.
Alternate Names: Uloric, Adenuric, Zurig, Febuxostatum
CAS No: 144060-53-7
PubChem CID: 134018
Mol Formula: C16H16N2O3S
Mol Weight: 316.38 g/mol
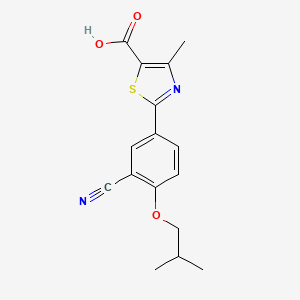
IUPAC Name: 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methyl-1,3-thiazole-5-carboxylic acid
API Description: Febuxostat is a non-purine xanthine oxidase (XO) inhibitor. In early 2008, febuxostat was granted marketing authorization by the European Commission for the treatment of chronic hyperuricemia and gout. In the following year, the FDA for approved febuxostat for use in the chronic management of hyperuricemia in adult patients with gout who have an inadequate response or intolerance to [allopurinol]. Gout is a form of arthritis that is caused by the accumulation of uric acid crystal in or around a joint, leading to inflammation and further deposition of uric acid crystal deposition in bones, joints, tissues, and other organs in the long term. Gout is closely associated with hyperuricemia. Febuxostat works by inhibiting the activity of an enzyme that is responsible for the synthesis of uric acid, thereby reducing serum uric acid levels. In February 2019, a black box warning for febuxostat was added, based on the findings of a post-market clinical study (the CARES trial) where there was an increased risk of cardiovascular (CV) fatal outcomes in patients with gout and known cardiovascular disease treated with febuxostat, when compared to those treated with allopurinol. The manufacturer and the FDA advise health professionals to limit the use of febuxostat to second-line therapy in patients who have inadequate response or intolerance to allopurinol, and to avoid the use of febuxostat in patients with cardiovascular diseases.