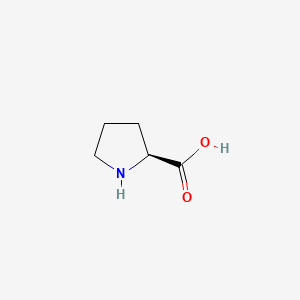
L-Proline (CAS No: 147-85-3) is a key pharmaceutical intermediate used in the synthesis of APIs Captopril and Vildagliptin. This compound serves as an essential raw material in API development and synthesis. Explore detailed chemical properties, molecular data, structure information, and verified global suppliers, manufacturers, and distributors of this API intermediate on apicule.
Alternate Names: proline, L-(-)-Proline, (2S)-pyrrolidine-2-carboxylic acid
CAS No: 147-85-3
PubChem CID: 145742
Mol Formula: C5H9NO2
Mol Weight: 115.13 g/mol
Used in APIs: Captopril and Vildagliptin
IUPAC Name: (2S)-pyrrolidine-2-carboxylic acid
API Intermediate Description: Proline is one of the twenty amino acids used in living organisms as the building blocks of proteins. Proline is sometimes called an imino acid, although the IUPAC definition of an imine requires a carbon-nitrogen double bond. Proline is a non-essential amino acid that is synthesized from glutamic acid. It is an essential component of collagen and is important for proper functioning of joints and tendons. L-proline is pyrrolidine in which the pro-S hydrogen at position 2 is substituted by a carboxylic acid group. L-Proline is the only one of the twenty DNA-encoded amino acids which has a secondary amino group alpha to the carboxyl group. It is an essential component of collagen and is important for proper functioning of joints and tendons. It also helps maintain and strengthen heart muscles. It has a role as a micronutrient, a nutraceutical, an algal metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a mouse metabolite and a member of compatible osmolytes. It is a glutamine family amino acid, a proteinogenic amino acid, a proline and a L-alpha-amino acid. It is a conjugate base of a L-prolinium. It is a conjugate acid of a L-prolinate. It is an enantiomer of a D-proline. It is a tautomer of a L-proline zwitterion.