Find verified Lazertinib API manufacturers, suppliers, and distributors across the globe. Explore companies that offer high-purity Lazertinib for pharmaceutical use, along with product details, certifications, and reliable sourcing options. Compare prices, check availability, and connect directly with trusted suppliers for Lazertinib API.
Alternate Names: Lazcluze, lazertinibum
CAS No: 1903008-80-9
PubChem CID: 121269225
Mol Formula: C30H34N8O3
Mol Weight: 554.6 g/mol
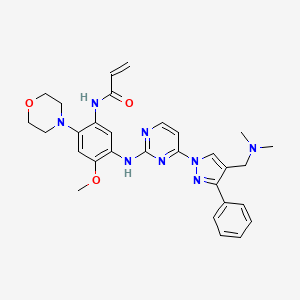
IUPAC Name: N-[5-[[4-[4-[(dimethylamino)methyl]-3-phenylpyrazol-1-yl]pyrimidin-2-yl]amino]-4-methoxy-2-morpholin-4-ylphenyl]prop-2-enamide
API Description: Lazertinib is an oral, third-generation, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI). Lazertinib was first approved in South Korea on January 18, 2021, for the treatment of EGFR T790M mutation-positive non-small cell lung cancer (NSCLC) with EGFR mutations. It was approved by the FDA on August 19, 2024. Lazertinib is used alone or in combination with other chemotherapeutic agents.