Find verified Mobocertinib API manufacturers, suppliers, and distributors across the globe. Explore companies that offer high-purity Mobocertinib for pharmaceutical use, along with product details, certifications, and reliable sourcing options. Compare prices, check availability, and connect directly with trusted suppliers for Mobocertinib API.
Alternate Names: EGFR/HER2 Inhibitor TAK-788
CAS No: 1847461-43-1
PubChem CID: 118607832
Mol Formula: C32H39N7O4
Mol Weight: 585.7 g/mol
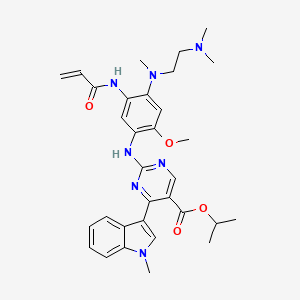
IUPAC Name: propan-2-yl 2-[4-[2-(dimethylamino)ethyl-methylamino]-2-methoxy-5-(prop-2-enoylamino)anilino]-4-(1-methylindol-3-yl)pyrimidine-5-carboxylate
API Description: Mobocertinib is a kinase inhibitor targeted against human epidermal growth factor receptor (EGFR). It is used specifically in the treatment of non-small cell lung cancer (NSCLC) caused by exon 20 insertion mutations in the EGFR gene, which are typically associated with a poorer prognosis (as compared to "classical" EGFR mutants causing NSCLC) and are associated with resistance to standard targeted EGFR inhibitors. Mobocertinib appears to be an effective means of treating this otherwise treatment-resistant NSCLC, exerting an inhibitory effect on EGFR exon 20 insertion mutant variants at concentrations 1.5- to 10-fold lower than those required to inhibit wild-type EGFR. Mobocertinib, under the brand name Exkivity (Takeda Pharmaceuticals Inc.), was granted accelerated approval by the FDA in September 2021 for the treatment of locally advanced or metastatic NSCLC in patients with EGFR exon 20 insertion mutations who have failed previous therapies.