Find verified Belumosudil API manufacturers, suppliers, and distributors across the globe. Explore companies that offer high-purity Belumosudil for pharmaceutical use, along with product details, certifications, and reliable sourcing options. Compare prices, check availability, and connect directly with trusted suppliers for Belumosudil API.
Alternate Names: Rock2 inhibitor kd025, Kinome_2597
CAS No: 911417-87-3
PubChem CID: 11950170
Mol Formula: C26H24N6O2
Mol Weight: 452.5 g/mol
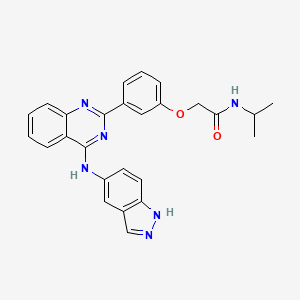
IUPAC Name: 2-[3-[4-(1H-indazol-5-ylamino)quinazolin-2-yl]phenoxy]-N-propan-2-ylacetamide
API Description: Belumosudil is used in the treatment of chronic graft-versus-host disease (GVHD) and has been investigated for the treatment of pulmonary arterial hypertension. It is an inhibitor of rho-associated coiled-coil-containing protein kinases (ROCK), with significantly more selectivity for ROCK2 as compared to ROCK1 (IC50 100 nM vs. 3 μM, respectively). In the treatment of GVHD, a condition in which donor T-cells begin to attack recipient tissues following allogeneic hematopoeitic stem cell transplantation (HSCT), belumosudil helps to resolve immune dysregulation by shifting the balance between Th17 cells and T-regulatory cells, thereby dampening the inflammatory cascade that can occasionally be fatal. Belumosudil was first approved by the FDA in July 2021, under the brand name Rezurock, for the treatment of chronic GVHD in patients who have tried and failed at least two prior lines of systemic therapy. In July 2022, Belumosudil was approved by Health Canada under the brand name RHOLISTIQ to treat the same condition in adult and pediatric patients 12 years or older.